VORTA for
Waste Water
Treatment Plants
An Advanced oxidation reactor leveraging OH•
VORTA harnesses the rotational flow which creates a unique possibility to modulate the shear attributed to the collapsing cavities to minimize damage to the biomolecules of interest
VORTA for
Waste Water
Treatment Plants
An Advanced oxidation reactor leveraging OH•
VORTA harnesses the rotational flow which creates a unique possibility to modulate the shear attributed to the collapsing cavities to minimize damage to the biomolecules of interest
VORTA RETROFIT
FOR ADVANCED OXIDATION
A Worthwhile Opportunity
To Validate
Within every running industrial WWTPs, there exists is a big room to harness the chemical potential of OH. produced through chemical free retrofit of VORTA.
We have gathered meaningful hands-on experiences with VORTA’s lab scale trials and commercial installations in terms of understanding its efficacy on the nature of the pollutant, nature of functional groups, & its structure (aliphatic, aromatic ,etc.)
VORTA harnesses the rotational flow which creates a unique possibility to modulate the shear attributed to the collapsing cavities to minimize damage to the biomolecules of interest
Complexity in industrial WWTPs
The complexity in the Industrial WWTPs is mainly attributed to (a) variety of different types of pollutants, and (b) high probability of finding refractory pollutants that are difficult to remove with the conventional biological treatment. Therefore depending on the objective VORTA retrofit has multiple value propositions in WWTPs
Variable Value Propositions of VORTA in WWT

COD Reduction
Reduction up to 20 to 60% ; Total COD as well Suspended COD can be targeted as an objective
Color Reduction
5-60% Color Reduction Possible, allows for a recycle possibility from a quality perspective
NH3-N Reduction
Up to 40-95% Reduction
In Ammonical Nitrogen, a toxic pollutant, is possible.
Selective Degradation
VORTA generates OH● in situ using cavitation allowing degradation of the Refractory pollutants
Complexity in industrial WWTPs
The complexity in the Industrial WWTPs is mainly attributed to (a) variety of different types of pollutants, and (b) high probability of finding refractory pollutants that are difficult to remove with the conventional biological treatment. Therefore depending on the objective VORTA retrofit has multiple value propositions in WWTPs
Variable Value Propositions of VORTA in WWT
COD
Recuction
Reduction up to 20 to 60% ; Total COD as well Suspended COD can be targeted as an objective
Color Reduction
5-60% Color Reduction Possible, allows for a recycle possibility from a quality perspective
NH3-N Reduction
Up to 40-95% Reduction
In Ammonical Nitrogen, a toxic pollutant, is possible.
Selective Degradation
VORTA generates OH● in situ using cavitation allowing degradation of the Refractory pollutants
Complexity in industrial WWTPs
The complexity in the Industrial WWTPs is mainly attributed to (a) variety of different types of pollutants, and (b) high probability of finding refractory pollutants that are difficult to remove with the conventional biological treatment. Therefore depending on the objective VORTA retrofit has multiple value propositions in WWTPs
Variable Value Propositions of VORTA
in WWT
COD Reduction
Reduction up to 20 to 60% ; Total COD as well Suspended COD can be targeted as an objective
Color Reduction
5-60% Color Reduction Possible, allows for a recycle possibility from a quality perspective
NH3-N Reduction
Up to 40-95% Reduction
In Ammonical Nitrogen, a toxic pollutant, is possible.
Selective Degradation
VORTA generates OH● in situ using cavitation allowing degradation of the Refractory pollutants
Persistent Pollutants
Persistent pollutants in wastewater refer to substances that are resistant to degradation and persist in the environment for extended periods. Examples of persistent pollutants include polycyclic aromatic hydrocarbons (PAHs), Polychlorinated biphenyls (PCBs), Dioxins, Polybrominated diphenyl ethers (PBDEs) , Dichlorodiphenyltrichloroethane (DDT) , and Pharmaceuticals and Personal Care Products (PPCPs). It's important to note that the presence and persistence of these pollutants in wastewater depend on various factors such as the sources of wastewater, industrial activities, treatment processes employed, and regulatory measures in place.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of synthetic chemicals that are difficult to degrade due to Strong Carbon-Fluorine Bonds making them highly resistant to chemical and biological degradation processes. The use of PFOA, perfluorooctanic acid is banned in the EU and the use of PFOS , perfluorooctane sulfonic acid, is only allowed for a few applications in the EU.
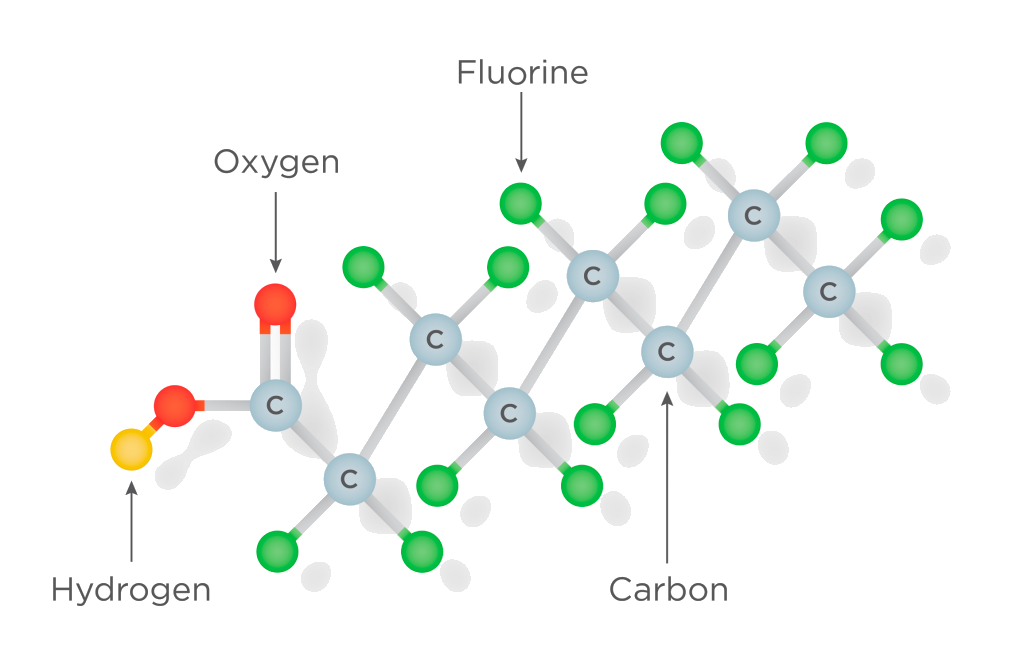
Cavitation as an alternative for treating Persistent Pollutants
Some studies have shown promising results in using cavitation to enhance the degradation of certain PFAS compounds in laboratory settings. In additional to OH radicals produced from cavitating bubbles, the high temperatures and pressures created during bubble collapse can induce pyrolysis or thermal degradation of PFAS compounds. Cavitation may not be equally effective for all types of PFAS compounds, and the extent of degradation achieved may vary but the there exists an opportunity to explore the potential of VORTA to degrade persistent pollutants
Persistent Pollutants
Persistent pollutants in wastewater refer to substances that are resistant to degradation and persist in the environment for extended periods. Examples of persistent pollutants include polycyclic aromatic hydrocarbons (PAHs), Polychlorinated biphenyls (PCBs), Dioxins, Polybrominated diphenyl ethers (PBDEs) , Dichlorodiphenyltrichloroethane (DDT) , and Pharmaceuticals and Personal Care Products (PPCPs). It's important to note that the presence and persistence of these pollutants in wastewater depend on various factors such as the sources of wastewater, industrial activities, treatment processes employed, and regulatory measures in place.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of synthetic chemicals that are difficult to degrade due to Strong Carbon-Fluorine Bonds making them highly resistant to chemical and biological degradation processes. The use of PFOA, perfluorooctanic acid is banned in the EU and the use of PFOS , perfluorooctane sulfonic acid, is only allowed for a few applications in the EU.

Cavitation as an alternative for treating Persistent Pollutants

Persistent Pollutants
Persistent pollutants in wastewater refer to substances that are resistant to degradation and persist in the environment for extended periods. Examples of persistent pollutants include polycyclic aromatic hydrocarbons (PAHs), Polychlorinated biphenyls (PCBs), Dioxins, Polybrominated diphenyl ethers (PBDEs) , Dichlorodiphenyltrichloroethane (DDT) , and Pharmaceuticals and Personal Care Products (PPCPs). It's important to note that the presence and persistence of these pollutants in wastewater depend on various factors such as the sources of wastewater, industrial activities, treatment processes employed, and regulatory measures in place.
Per- and polyfluoroalkyl substances (PFAS)
PFAS are a group of synthetic chemicals that are difficult to degrade due to Strong Carbon-Fluorine Bonds making them highly resistant to chemical and biological degradation processes. The use of PFOA, perfluorooctanic acid is banned in the EU and the use of PFOS , perfluorooctane sulfonic acid, is only allowed for a few applications in the EU.


